Epigenetics, a captivating field of study, has revolutionised our understanding of gene regulation. While genetics focuses on the DNA sequence itself, epigenetics explores the intricate mechanisms that influence gene expression without altering the DNA sequence. These epigenetic changes can have a profound impact on an individual’s health, development, and susceptibility to diseases. In this comprehensive guide, we will delve into the fascinating types of epigenetic modifications, exploring its mechanisms, implications, and potential applications.
Understanding epigenetics: the key to unravelling gene regulation and expression
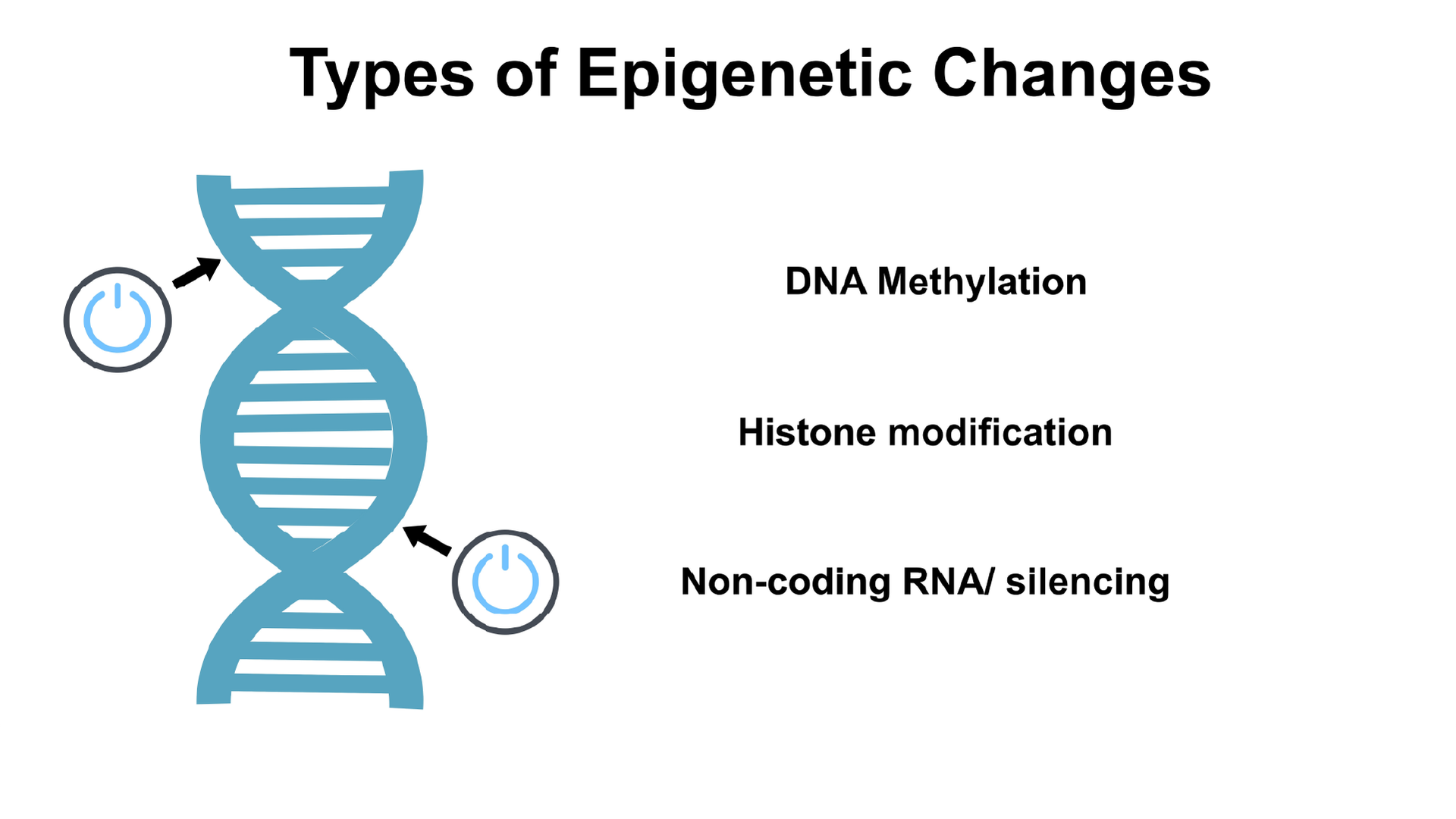
Types of epigenetic modifications: shaping gene expression
Different types of epigenetic modifications influence how genes are turned on and off, ultimately determining which proteins are produced. These modifications can occur through various mechanisms, such as DNA methylation, histone modifications, and RNA-associated silencing.
DNA methylation: DNA methylation involves the addition of a methyl group to specific regions of the DNA, known as CpG sites. This chemical modification can inhibit gene expression by preventing the binding of proteins that activate gene transcription.
Histone modifications: Histones, proteins that package DNA into a compact structure called chromatin, can undergo modifications that alter the accessibility of genes. Acetylation and methylation of histones can either promote or suppress gene expression, depending on the specific modifications and their location.
RNA-associated silencing: RNA molecules can also be involved in silencing gene expression. Non-coding RNAs and RNA interference can bind to messenger RNA (mRNA) molecules, leading to their degradation or preventing their translation into proteins.
The epigenome: a complex landscape of epigenetic changes
The collection of all types of epigenetic modifications in an organism’s genome is referred to as the epigenome. It acts as a dynamic blueprint that regulates gene expression and cellular identity. The epigenome is influenced by a combination of genetic and environmental factors, playing a crucial role in development, ageing, and disease.
Genetic factors: Certain genetic variations can impact the establishment and maintenance of epigenetic marks, highlighting the intricate interplay between genetics and epigenetics.
Environmental influences: Environmental factors, such as diet, stress, and exposure to toxins, can profoundly shape the epigenome. These influences can be transient or have long-lasting effects, potentially affecting future generations.
Unravelling the impact of epigenetics: from development to disease
Developmental epigenetics: orchestrating cellular diversity
During development, the different types of epigenetic modifications play a pivotal role in determining cell fate and orchestrating the remarkable diversity of cell types in our bodies. Despite all cells containing the same genetic material, they exhibit distinct patterns of gene expression, leading to their specialised functions and characteristics.
Cellular reprogramming: The ability to reprogram cells to assume different identities has revolutionised regenerative medicine. Epigenetic modifications are at the core of cellular reprogramming, allowing scientists to convert one cell type into another, offering new avenues for tissue regeneration and personalised medicine.
Imprinting and X-chromosome inactivation: Epigenetics also governs genomic imprinting, a process through which specific genes are preferentially expressed based on their parental origin. Additionally, X-chromosome inactivation ensures the equal expression of X-linked genes in females, compensating for the presence of two X chromosomes.
Epigenetics and disease: unravelling the molecular basis
Epigenetic dysregulation can contribute to various diseases, including cancer, neurodevelopmental disorders, and age-related conditions. Understanding the molecular mechanisms underlying these diseases provides valuable insights into diagnosis, treatment, and prevention strategies.
Cancer and epigenetics: Epigenetic alterations are a hallmark of cancer, often affecting critical genes involved in cell growth, division, and DNA repair. Aberrant DNA methylation and histone modifications can lead to the silencing of tumour-suppressor genes or the activation of oncogenes, driving tumour development.
Neurodevelopmental disorders: Epigenetic changes have also been implicated in neurodevelopmental disorders, such as autism spectrum disorders and intellectual disabilities. Disruptions in epigenetic regulation during brain development can significantly impact neuronal function and synaptic connectivity.
Age-related epigenetic changes: Epigenetic modifications accumulate over time, contributing to the ageing process. These age-related changes can affect gene expression patterns, leading to functional decline and increased susceptibility to age-related diseases.
The power of epigenetic data: epigenetic analysis techniques
Types of epigenetic data
Epigenetic data encompasses a range of information that provides insights into the various modifications occurring within an individual’s genome. These include DNA methylation profiles, histone modification patterns, and non-coding RNA expression levels.
DNA methylation profiles: DNA methylation patterns can be assessed using techniques such as enzymatic- or bisulfite-based sequencing or DNA methylation arrays. These approaches allow researchers to identify specific regions of the genome that are methylated or demethylated, shedding light on gene regulatory processes.
Histone modification patterns: Chromatin immunoprecipitation sequencing (ChIP-seq) enables the mapping of histone modifications across the genome, providing a comprehensive understanding of their distribution and association with gene expression.
Non-coding RNA expression: RNA sequencing (RNA-seq) allows for the profiling of non-coding RNA molecules, providing insights into their role in gene regulation and cellular processes.
Epigenetic data extraction and analysis
Extracting and analysing epigenetic data requires sophisticated techniques and computational tools. High-throughput sequencing technologies, combined with bioinformatics approaches, enable the generation and interpretation of vast amounts of epigenetic data.
High-throughput sequencing: Next-generation sequencing technologies have revolutionised the field of epigenomics, allowing researchers to generate comprehensive datasets that capture the complexity of epigenetic modifications.
Bioinformatics analysis: Advanced bioinformatics algorithms and pipelines are employed to analyse epigenetic data, including processing raw sequencing reads, mapping them to the reference genome, and identifying significant epigenetic features, such as differentially methylated regions or histone modification peaks.
Applications of epigenetic data
Epigenetic data holds immense potential for various fields, including cancer research, ageing studies, and precision medicine. These data provide valuable insights into disease mechanisms, biomarker discovery, and the development of targeted therapies.
Cancer research: Epigenetic alterations are being extensively studied in cancer research, offering opportunities for early detection, prognostication, and therapeutic interventions. Epigenetic biomarkers can aid in cancer diagnosis, guiding treatment decisions, and monitoring treatment response.
Ageing and age-related diseases: Epigenetic changes play a significant role in the ageing process and age-related diseases. Studying age-related epigenetic modifications can provide insights into the mechanisms underlying ageing and potentially identify interventions to promote healthy ageing.
Precision medicine: Epigenetic data can contribute to the development of personalised medicine approaches. By profiling epigenetic modifications in individuals, clinicians can tailor treatment strategies based on an individual’s unique epigenetic profile, enhancing treatment efficacy and minimising adverse effects.Explore the fascinating world of the different types of epigenetic modifications and the role of epigenetics in gene expression. Learn more.
Conclusion: unveiling the epigenetic landscape
Epigenetics has revolutionised our understanding of gene regulation, shedding light on the intricate mechanisms that shape our development, health, and susceptibility to diseases. The dynamic nature of the epigenome, influenced by genetic and environmental factors, highlights the complexity of gene regulation. Epigenetic data, with its wealth of information, provides valuable insights into disease mechanisms, personalised medicine, and the potential for targeted interventions. As we continue to unravel the secrets of epigenetics, we open new avenues for improving human health and unlocking the full potential of our genomes.